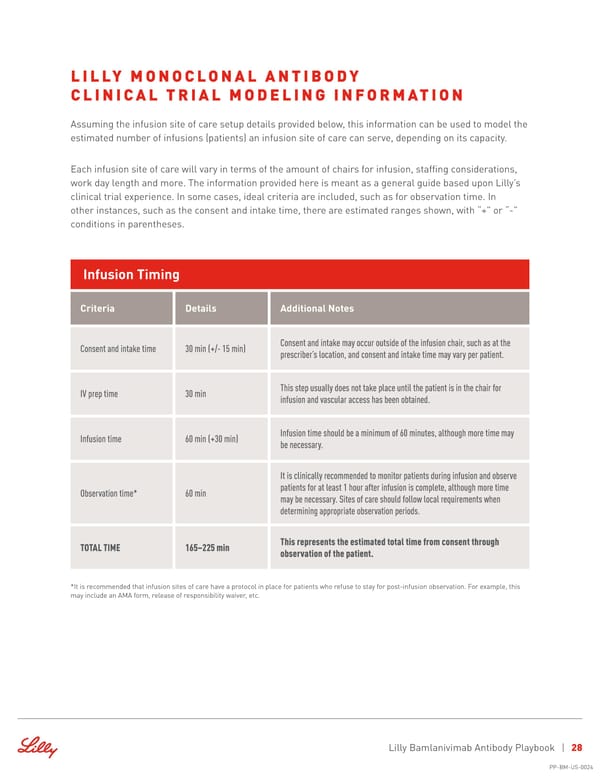
LILLY MONOCLONAL ANTIBODY CLINICAL TRIAL MODELING INFORMATION Assuming the infusion site of care setup details provided below, this information can be used to model the estimated number of infusions (patients) an infusion site of care can serve, depending on its capacity. Each infusion site of care will vary in terms of the amount of chairs for infusion, staffing considerations, work day length and more. The information provided here is meant as a general guide based upon Lilly’s clinical trial experience. In some cases, ideal criteria are included, such as for observation time. In other instances, such as the consent and intake time, there are estimated ranges shown, with “+” or “-” conditions in parentheses. Infusion Timing Criteria Details Additional Notes Consent and intake time 30 min (+/- 15 min) Consent and intake may occur outside of the infusion chair, such as at the prescriber’s location, and consent and intake time may vary per patient. IV prep time 30 min This step usually does not take place until the patient is in the chair for infusion and vascular access has been obtained. Infusion time 60 min (+30 min) Infusion time should be a minimum of 60 minutes, although more time may be necessary. It is clinically recommended to monitor patients during infusion and observe Observation time* 60 min patients for at least 1 hour after infusion is complete, although more time may be necessary. Sites of care should follow local requirements when determining appropriate observation periods. TOTAL TIME 165–225 min This represents the estimated total time from consent through observation of the patient. *It is recommended that infusion sites of care have a protocol in place for patients who refuse to stay for post-infusion observation. For example, this may include an AMA form, release of responsibility waiver, etc. Lilly Bamlanivimab Antibody Playbook | 28 PP-BM-US-0024
 Antibody Playbook Page 27 Page 29
Antibody Playbook Page 27 Page 29