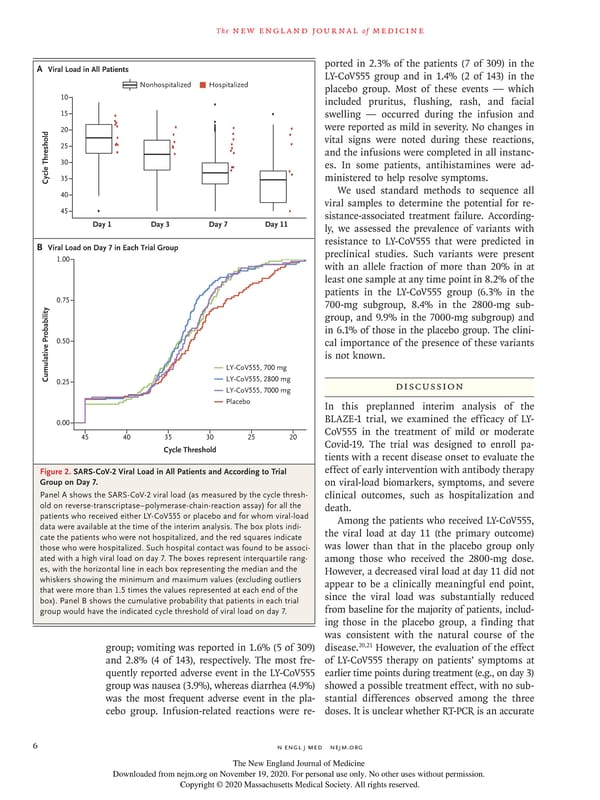
The new england journal of medicine Viral Load in ll Patients ported in 2.3% of the patients (7 of 309) in the LY-CoV555 group and in 1.4% (2 of 143) in the Nonhospitalized Hospitalized placebo group. Most of these events — which 10 included pruritus, flushing, rash, and facial 15 swelling — occurred during the infusion and 20 were reported as mild in severity. No changes in vital signs were noted during these reactions, 25 and the infusions were completed in all instanc- 30 es. In some patients, antihistamines were ad- Cycle Threshold35 ministered to help resolve symptoms. 40 We used standard methods to sequence all viral samples to determine the potential for re- 45 sistance-associated treatment failure. According- Day 1 Day 3 Day 7 Day 11 ly, we assessed the prevalence of variants with B Viral Load on Day 7 in Each Trial Group resistance to LY-CoV555 that were predicted in 1.00 preclinical studies. Such variants were present with an allele fraction of more than 20% in at least one sample at any time point in 8.2% of the patients in the LY-CoV555 group (6.3% in the 0.75 700-mg subgroup, 8.4% in the 2800-mg sub- group, and 9.9% in the 7000-mg subgroup) and in 6.1% of those in the placebo group. The clini- 0.50 cal importance of the presence of these variants is not known. LY-CoV555,700 Cumulative Probability0.25 LY-CoV555,200 LY-CoV555,7000 Discussion Placebo In this preplanned interim analysis of the 0.00 BLAZE-1 trial, we examined the efficacy of LY- 45 40 35 30 25 20 CoV555 in the treatment of mild or moderate Cycle Threshold Covid-19. The trial was designed to enroll pa- tients with a recent disease onset to evaluate the Figure 2. SARS-CoV-2 Viral Load in All Patients and According to Trial effect of early intervention with antibody therapy Group on Day 7. on viral-load biomarkers, symptoms, and severe Panel A shows the SARS-CoV-2 viral load (as measured by the cycle thresh- clinical outcomes, such as hospitalization and old on reverse-transcriptase–polymerase-chain-reaction assay) for all the death. patients who received either LY-CoV555 or placebo and for whom viral-load Among the patients who received LY-CoV555, data were available at the time of the interim analysis. The box plots indi- the viral load at day 11 (the primary outcome) cate the patients who were not hospitalized, and the red squares indicate those who were hospitalized. Such hospital contact was found to be associ- was lower than that in the placebo group only ated with a high viral load on day 7. The boxes represent interquartile rang- among those who received the 2800-mg dose. es, with the horizontal line in each box representing the median and the However, a decreased viral load at day 11 did not whiskers showing the minimum and maximum values (excluding outliers appear to be a clinically meaningful end point, that were more than 1.5 times the values represented at each end of the since the viral load was substantially reduced box). Panel B shows the cumulative probability that patients in each trial group would have the indicated cycle threshold of viral load on day 7. from baseline for the majority of patients, includ- ing those in the placebo group, a finding that was consistent with the natural course of the 20,21 group; vomiting was reported in 1.6% (5 of 309) disease. However, the evaluation of the effect and 2.8% (4 of 143), respectively. The most fre- of LY-CoV555 therapy on patients’ symptoms at quently reported adverse event in the LY-CoV555 earlier time points during treatment (e.g., on day 3) group was nausea (3.9%), whereas diarrhea (4.9%) showed a possible treatment effect, with no sub- was the most frequent adverse event in the pla- stantial differences observed among the three cebo group. Infusion-related reactions were re- doses. It is unclear whether RT-PCR is an accurate 6 n engl j med nejm.org The New England Journal of Medicine Downloaded from nejm.org on November 19, 2020. For personal use only. No other uses without permission. Copyright © 2020 Massachusetts Medical Society. All rights reserved.
 NEJM - Neutralizing Antibody LY-COV555 Page 5 Page 7
NEJM - Neutralizing Antibody LY-COV555 Page 5 Page 7