Integrated Summary Report
42 pages

1 2019 INTEGRATED SUMMARY REPORT Lilly unites caring with discovery to create medicines that make life better for people around the world.

2 ELI LILLY AND COMPANY To our Lilly shareholders exposure, upcoming data readouts for key growth drivers, and additional potential approvals and productivity improvements in the works, it is an exciting time for Lilly and the people we serve. DELIVERING RESULTS Thanks to the work of our more than 33,000 employees, Lilly’s revenue grew 4% to $22.3 billion in 2019, driven by volume. Despite the lingering impact of the loss of exclusivity for Cialis, sales volume rose 8% while net selling price declined 3%. Our key growth products, including Trulicity, Taltz, Verzenio, Jardiance and Emgality, continued to drive impressive worldwide volume growth. During the past year, we’ve made strides toward leadership in each I am pleased to report that 2019 was a strong year for Eli Lilly of our therapeutic research areas: diabetes, oncology, immunology, and Company. We delivered solid financial results, developed and pain and neurodegeneration. We continued our unprecedented launched important new medicines, and made progress on our pace of launching new medicines and improved manufacturing productivity agenda. The most meaningful metric for Lilly – productivity. We’re on track to meet our goal of 31% non-GAAP the number of people our medicines are helping – totaled more operating margin by the end of 2020, even as we increase than 40 million in the past year alone. investments in R&D. While this figure is remarkable, the ease with which people can To this end, we are focused on strengthening the efficiency of our access innovative medicines varies widely around the world. R&D engine. 2019 produced significant advancements, as we: In the U.S., broad access to the latest treatments can be constrained by complicated or narrow insurance benefits that • received U.S. approval for two new medicines, Reyvow reduce affordability. In other advanced economies, countries and Baqsimi; sometimes ration new treatments to fund the obligations of government-run health programs. And in developing countries, • obtained new indication approvals for Trulicity, Taltz, nascent health systems struggle to allocate limited resources Emgality and Cyramza; between care for acute and chronic diseases. • added four new Phase 3 clinical programs to our pipeline, These issues are complex, but we have solved difficult challenges all with potential to be first-in-class or best-in-class; before. As a company that has been in business for 144 years and invests more than $5 billion annually in research and development • reported 12 positive Phase 3 or registrational trial read- (R&D), we are in this for the long haul. We understand the outs, including a mix of new molecular entities (NMEs), importance of adapting, evolving and improving while remaining new indications or data for launched products; and firmly grounded in our core values: integrity, excellence and respect • submitted 12 NMEs or new indications for regulatory for people. With these standards as our guide, we will continue to review in geographies around the world. find effective ways to partner within and across health systems with the aim of facilitating patient access to the latest treatments, regardless of income level or geography. In 2019, Lilly invested more than $13 billion to drive our We have made progress, but we know there is still much to do. future growth through a combination of business development, As we transition to a new decade, our pipeline and commercial capital expenditures and after-tax investment in R&D. successes will serve as a springboard for sustained growth and We returned approximately $7 billion to shareholders via productivity. With strong new product growth and limited patent dividends and share repurchases and announced a 15%
3 2019 INTEGRATED SUMMARY REPORT dividend increase for the second consecutive year. And over LOOKING AHEAD the past five years, our annualized total shareholder return has 2020 is shaping up to be another exciting year at Lilly. We expect averaged 16.7%, compared to 11.7% for the S&P benchmark. to achieve high single-digit revenue growth, exceeding our five-year goals, with more than half of our sales driven by volume of our SUSTAINING PROGRESS newer products. We remain committed to further margin expansion From our company’s earliest days, we have actively engaged with while we continue to invest in our innovation-based strategy. our partners in health systems around the world to ensure that our In addition to Reyvow for the acute treatment of migraine, we medicines can reach the people who need them. One way we do expect to launch two more new medicines this year: URLi, a that is through value-based contracts, in which the price we receive fast-acting mealtime insulin for diabetes, and selpercatinib for depends on how much our medicines help patients. In the U.S., non-small cell lung cancer and thyroid cancers. Selpercatinib, part 20% of revenue flowing through access-based contracts has a of our 2019 acquisition of Loxo Oncology, shrank tumors in the value-based component, and we have more than 300 alternative vast majority of study participants, like Tanner Noble, whom you access contracts in other global markets, many of which are can read more about on the next page. value-based. In the U.S., our success in working with payers and government We’re closely tracking the potential breakthroughs represented entities to expand access to insulin and other therapies has been by other late-phase assets, including tirzepatide, which has tempered by the realization that the widening difference between generated encouraging data for blood glucose control and list and net prices is not sustainable. Of particular concern are weight loss in people with diabetes, and mirikizumab, which high-deductible insurance plans, which can financially burden we’re studying in ulcerative colitis, Crohn’s disease and psoriasis. patients with chronic diseases. For people who use insulin, we’ve Our early phase pipeline provides a good foundation for future launched lower-priced versions of Humalog, complementing our growth, highlighted by new cancer medicines such as our comprehensive patient assistance programs that seek to lower BTK inhibitor (LOXO-305) and our KRAS G12C inhibitor. out-of-pocket cost for people with diabetes like Doug Liebman. Given the life-changing potential of medicines, we consider You can read his story on page 20. breakthroughs that lead to greater access just as critical as those While these programs are helping, it’s clear that broader, that lead to innovative treatments. We bring a strong sense of system-wide changes are needed to shift costs away from patients. urgency to ensuring that our medicines are available and A potential first step in resetting the financial incentives for each affordable for patients who need them. entity in the pharmaceutical supply chain would be to increase the As we enter the next decade, we see an exceptional opportunity transparency of net pricing and explore its use as the basis for all to create new standards of care and accessibility in some of the transactions, including patient cost sharing. The Transparency world’s most serious diseases, advance the boundaries of possibility section of this report contains further insight into how we’re in biopharmaceuticals, and deliver extraordinary value to patients, addressing this issue, especially for people with diabetes. other health care customers, shareholders, employees and In Europe and Japan, we’re partnering with governments to gather communities. We will do all we can to realize that potential. evidence of our medicines’ value to support appropriate use and With sincere appreciation for your ongoing interest and support, a fair price. We’re accelerating availability, cutting months to reimbursement in major European markets by more than 50% since 2017. In developing countries, we’re actively enhancing existing health systems while supplying our most essential products in ways that ensure affordable access. You can read more about our important initiatives to improve global health, including Lilly 30x30 and our health worker training program, in the Corporate Responsibility section on page 26. David A. Ricks, Chairman and CEO
4 ELI LILLY AND COMPANY LIZ MCFADDIN SCIENTIST, LOXO ONCOLOGY AT LILLY Liz McFaddin with Tanner Noble, thyroid cancer survivor Tanner Noble should have been a growing boy. But as he began his 8th grade football season, the 14-year old couldn’t keep food down and kept shrinking. He lost nearly 100 pounds. For a year, doctors couldn’t tell him why. Finally, he received a diagnosis of thyroid cancer and began chemotherapy. But Tanner struggled, and nothing his doctors tried worked. His parents watched him waste away, preparing themselves for the end. Then his oncologist at Cleveland Clinic helped him enroll in a clinical study of an experimental precision cancer medicine developed by Loxo Oncology. Within a month, Tanner’s feeding tube was removed, and he was back to eating chicken parmesan, his favorite meal. Now 18, he’s a freshman in college, inspiring his Ohio hometown that rallied to his cause. “What I want to do in life is write my story and bring the same gift that people have given to me,” Tanner said, “and that’s another chance at life.” Liz McFaddin, a medicinal chemist at Loxo Oncology at Lilly, understands the personal impact of her work. “Many of us have family members who have been affected by cancer, and we know they deserve better,” Liz said. “But I didn’t know it was going to lead to Tanner.” Liz recently met Tanner and his mother, Demetra, during a visit to Lilly’s headquarters in Indianapolis. “That’s why I come to work every day, because there’s a patient at the end of it. But I couldn’t have ever imagined meeting someone who was so dramatically affected by the work I contributed to,” Liz said. “I’m so happy that he gets to go and enjoy this time and go to college. It’s remarkable.”
5 2019 INTEGRATED SUMMARY REPORT “That’s why we come to work every day, because there’s a patient f it.” at the end o

6 ELI LILLY AND COMPANY “What I want to do in life is write my story and bring the same gift that people have given to me, and that’s another chance at life.” TANNER NOBLE THYROID CANCER SURVIVOR

7 2019 INTEGRATED SUMMARY REPORT SCIENCE OVERVIEW Speed Life-Changing Medicines Lilly has always pushed the boundaries of science in order to caused by specific gene mutations. One of these new medicines, make conditions that are incurable today, treatable tomorrow. selpercatinib, has been submitted to regulators for approval to treat In 2019, we received regulatory approval for two new medicines – medullary thyroid cancers and non-small cell lung cancer and Baqsimi, which launched last year, and Reyvow, which launched received U.S. priority review designation. this year. We’ve brought 11 new therapies to patients since 2014 With technology partner Strateos, we opened a first-of-its-kind, and expect to introduce nine more by the end of 2023, achieving robotic laboratory in San Diego. The Lilly Life Sciences Studio our goal of 20 new medicines in 10 years. physically and virtually integrates several areas of the drug Our pipeline continues to be cited as one of the most promising discovery process together into a fully automated platform. The lab in the industry across our core therapeutic areas of diabetes, enables research scientists to remotely control their experiments via oncology, immunology, pain and neurodegeneration. We’re moving a web-based interface. This technology was designed to decrease medicines to patients faster. The number of molecules starting the amount of time it takes scientists to receive critical data, thus clinical trials in 2019 rose by nearly 50% versus the previous year. accelerating the drug discovery process. To accomplish this, we’re using creative methods to access Lilly created a new shared innovation lab in South San Francisco, innovation – investing in advanced technologies and looking designed to speed the discovery of innovative medicines through beyond our walls for emerging scientific ideas. collaboration with other biotech companies. Lilly Gateway Labs An example of this new thinking is how we approach early phase provides companies the potential opportunity to collaborate with oncology research. We created Loxo Oncology at Lilly, a combined Lilly on projects of mutual interest and exposure to Lilly’s scientific organization that marries the speed and agility of Loxo with the and drug development expertise. They also have the potential for scale and capabilities of Lilly. We acquired Loxo Oncology in 2019, financial investment from Lilly, venture funds or both. adding potential medicines tailored for patients with cancers New Medicines for Patients Global Launches Potential Launches 2014 2015 2016 2017 2018 2019 2020 2021 2022 2023 Trulicity® Basaglar® Taltz® Verzenio® Emgality® Baqsimi™ Reyvow™ Tanezumab Tirzepatide BTK Inhibitor Jardiance® Portrazza® Olumiant® Ultra-Rapid Mirikizumab Lebrikizumab Cyramza® Lispro (IL-13 mAb) Selpercatinib Automated Insulin Delivery System RET Inhibitor Effort 2
8 PIPELINE ELI LILLY AND COMPANY Updated January 27, 2020 Phase 1 Phase 2 NME NME Angiopoietin 3/8 mAb CVD Automated Insulin Diabetes DACRA-089 Diabetes Delivery System GDF 15 Agonist Diabetes Basal Insulin-FC Diabetes GGG Tri-agonist Diabetes CD200R mAb Agonist Immunology GLP-1 Receptor NPA Diabetes D1 PAM Dementia Oxyntomodulin Diabetes Donanemab (N3pG Aβ mAb) Alzheimer’s GIP/GLP Co-agonist Peptide Diabetes Zagotenemab (Tau mAb) Alzheimer’s BAFF/IL-17 Bispecific Immunology NILEX BTLA mAb Agonist Immunology CXCR1/2L mAb Immunology Tirzepatide Nonalcoholic steatohepatitis IL-2 Conjugate Immunology (NASH) IL-33 mAb Immunology Olaratumab Pancreatic PD-1 mAb Agonist Immunology Cancer D1 PAM II Dementia Abemaciclib Prostate Cancer O-GlcNAcase Inhibitor Alzheimer’s Tau Morphomer Alzheimer’s Aurora A Kin Inhibitor Cancer BTK Inhibitor Cancer Cancer (undisclosed) Cancer CD226 Agonist Cancer ERK Inhibitor Cancer KRAS G12C Inhibitor Cancer PD-1/PD-L1 Bispecific Cancer SERD Cancer PACAP38 mAb Pain SSTR4 Agonist Pain TrpA1 Antagonist Pain KEY ADVANCES IN OUR INNOVATION STRATEGY Tirzepatide Lebrikizumab A dual GIP and GLP-1 receptor agonist with the potential for An IL-13 antibody added via the acquisition of Dermira joins game-changing reductions in A1C and body weight in people baricitinib, an oral JAK 1/JAK 2 inhibitor, as a second approach with type 2 diabetes. It’s also being studied in obesity and NASH, being studied to help people suffering from atopic dermatitis. an inflammatory condition caused by liver fat.
9 2019 INTEGRATED SUMMARY REPORT Phase 3 Regulatory Review NME NME Tirzepatide Diabetes Ultra-Rapid Lispro Diabetes Lebrikizumab (IL-13 mAb) Atopic Flortaucipir Tau Imaging, Dermatitis diagnostic Mirikizumab Psoriasis Selpercatinib RET fusion & Solanezumab Alzheimer’s RET mutant cancers NILEX Tanezumab Osteoarthritis Pain Empagliflozin Chronic Kidney Disease NILEX Empagliflozin Heart Failure Dulaglutide 3.0/4.5 mg Diabetes Tirzepatide Obesity Connected Care Diabetes Baricitinib Alopecia Areata Prefilled Insulin Pen Baricitinib Systemic Lupus Empagliflozin Type 1 Diabetes Erythematosus Baricitinib Atopic Dermatitis Mirikizumab Ulcerative Colitis Ixekizumab Non-Radiographic Mirikizumab Crohn’s Disease AxSpA Abemaciclib Adjuvant Breast Ixekizumab Pediatric Psoriasis Cancer Selpercatinib 1L NSCLC Selpercatinib 1L Medullary Thyroid Cancer Tanezumab Cancer Pain DIABETES IMMUNOLOGY NEURODEGENERATION ONCOLOGY PAIN Ultra-Rapid Lispro (URLi) Mirikizumab A novel, fast-acting mealtime insulin lispro for adults with type 1 and An IL-23 antibody that could transform the treatment of ulcerative type 2 diabetes. It’s designed to more closely mirror the way insulin colitis and Crohn’s disease and is also being studied to advance the works in people without diabetes. Lilly has received a positive opinion treatment of psoriasis. from regulators in Europe and has submitted URLi for regulatory approval in the U.S. and Japan.
10 ELI LILLY AND COMPANY JEFF YANG GLOBAL DEVELOPMENT LEADER, MIGRAINE For Lilly’s migraine team, pushing the boundaries of science started with asking bold questions. “We listened to patients and external advisors to learn how we could reset treatment expectations. Could we push ourselves to demonstrate the medicine can help patients be migraine free?” said Jyun-Yan (Jeff) Yang, M.D., global development leader for migraine. “It allowed us to design the Phase 3 studies to raise the bar on what a migraine treatment could deliver.” In 2019, that preventative migraine treatment – Emgality – became the U.S. leader in new-to-brand prescriptions and received FDA approval for episodic cluster headache. In 2020, Lilly launched a second new medicine, Reyvow, to stop migraine attacks when they happen – the first new class of acute migraine treatment approved by the FDA in more than two decades. “These new medicines are bringing new optimism for people living with migraine,” Dr. Yang said. Merle Diamond, M.D., president and managing director of the Diamond Headache Clinic in Chicago, said new classes of medication have made life better for her patients. “Having these medications has changed my 30-year practice of headache,” said Dr. Diamond. “These are the first doors that we have been able to walk through to help our patients achieve a life without disability.”
11 2019 INTEGRATED SUMMARY REPORT “A migraine attack doesn’t have to mean the end of a patient’s day. Being pain free is now the treatment goal.”

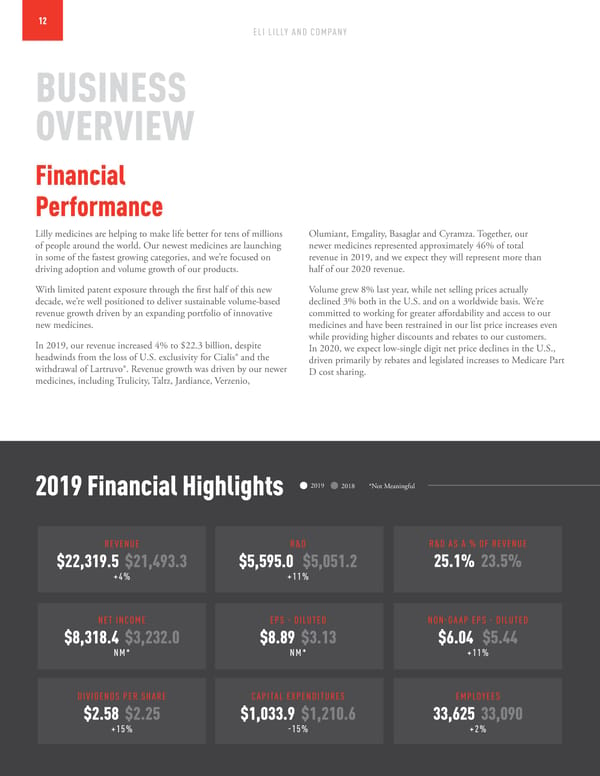
12 ELI LILLY AND COMPANY BUSINESS OVERVIEW Financial Performance Lilly medicines are helping to make life better for tens of millions Olumiant, Emgality, Basaglar and Cyramza. Together, our of people around the world. Our newest medicines are launching newer medicines represented approximately 46% of total in some of the fastest growing categories, and we’re focused on revenue in 2019, and we expect they will represent more than driving adoption and volume growth of our products. half of our 2020 revenue. With limited patent exposure through the first half of this new Volume grew 8% last year, while net selling prices actually decade, we’re well positioned to deliver sustainable volume-based declined 3% both in the U.S. and on a worldwide basis. We’re revenue growth driven by an expanding portfolio of innovative committed to working for greater affordability and access to our new medicines. medicines and have been restrained in our list price increases even In 2019, our revenue increased 4% to $22.3 billion, despite while providing higher discounts and rebates to our customers. headwinds from the loss of U.S. exclusivity for Cialis® and the In 2020, we expect low-single digit net price declines in the U.S., withdrawal of Lartruvo®. Revenue growth was driven by our newer driven primarily by rebates and legislated increases to Medicare Part medicines, including Trulicity, Taltz, Jardiance, Verzenio, D cost sharing. 2019 Financial Highlights 2019 2018 *Not Meaningful REVENUE R&D R&D AS A % OF REVENUE $22,319.5 $21,493.3 $5,595.0 $5,051.2 25.1% 23.5% +4% +11% NET INCOME EPS - DILUTED NON-GAAP EPS - DILUTED $8,318.4 $3,232.0 $8.89 $3.13 $6.04 $5.44 NM* NM* +11% DIVIDENDS PER SHARE CAPITAL EXPENDITURES EMPLOYEES $2.58 $2.25 $1,033.9 $1,210.6 33,625 33,090 +15% -15% +2%
13 2019 INTEGRATED SUMMARY REPORT We continue to advance our productivity agenda and control future growth opportunities, as we did with the acquisition of operating expenses while investing in key brands and our late-stage Loxo Oncology in 2019 and Dermira, Inc. in early 2020. pipeline. We made targeted, strategic investments across our Finally, we return cash to shareholders through our dividend, commercial portfolio and pipeline, which we believe will enhance which increased 15% in 2019 and will increase another 15% in our opportunities for future growth. We expect ongoing 2020, as well as through further share repurchases. productivity improvements that will lead to further operating margin expansion, and we’re on track to achieve our operating As we enter this new decade, we have an opportunity to margin target in 2020. deliver meaningful value to our shareholders, customers, We also delivered solid earnings per share growth and generated employees and communities by creating new standards of strong cash flow in 2019. Our capital allocation priorities remain care and advancing the boundaries of what’s possible in unchanged. We focus first on funding our promising pipeline and some of the world’s most serious diseases. our recently launched medicines. We then look to leverage business development to access external innovation and augment our RECONCILING ITEMS BETWEEN EPS-DILUTED AND NON-GAAP EPS-DILUTED Discontinued Operations from disposition of Elanco1 -3.93 -0.08 Amoritization of intangible assets 0.18 0.28 Asset impairment, restructuring, and other special 0.58 0.24 Charges related to withdrawal of Lartruvo 0.14 -- charges1 Impact of reduced shares outstanding for Gain on sale of China antibiotics business1 -0.26 -- non-GAAP reporting2 0.07 0.20 Charge related to repurchase of debt1 0.22 -- Income taxes3 -0.05 -0.27 Acquired in-process research and development1 0.21 1.96 Other, net -- -0.02 1 For more information on these reconciling items, see the Financial Results section of the Executive Overview in Management’s Discussion and Analysis in the company’s latest Form 10-K filed with the U.S. Securities and Exchange Commission. 2 Non-GAAP earnings per share assume that the disposition of Elanco occurred at the beginning of all periods presented and, therefore, exclude the approximately 65.0 million shares of Lilly common stock retired in the Elanco exchange offer. 3 For 2019, amount relates to a tax benefit from a capital loss on the disposition of subsidiary stock. For 2018, amount relates to adjustments to the 2017 Toll Tax for U.S. tax reform proposed regulations and tax expenses associated with the separation of Elanco. 4 Numbers may not add due to rounding.
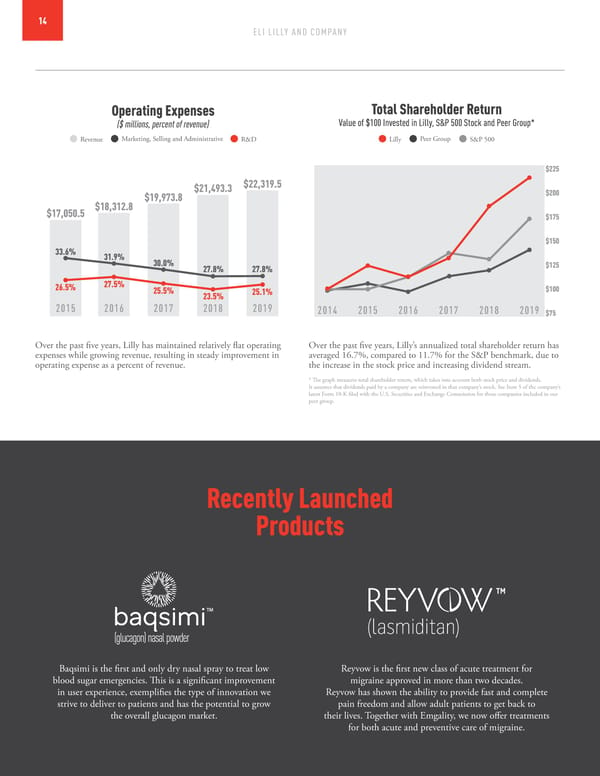
14 ELI LILLY AND COMPANY Operating Expenses Total Shareholder Return ($ millions, percent of revenue) Value of $100 Invested in Lilly, S&P 500 Stock and Peer Group* Revenue Marketing, Selling and Administrative R&D Lilly Peer Group S&P 500 $225 $21,493.3 $22,319.5 $19,973.8 $200 $17,050.5 $18,312.8 $175 $150 33.6% 31.9% 30.0% 27.8% 27.8% $125 26.5% 27.5% 25.5% 25.1% $100 23.5% 2015 2016 2017 2018 2019 2014 2015 2016 2017 2018 2019 $75 Over the past five years, Lilly has maintained relatively flat operating Over the past five years, Lilly’s annualized total shareholder return has expenses while growing revenue, resulting in steady improvement in averaged 16.7%, compared to 11.7% for the S&P benchmark, due to operating expense as a percent of revenue. the increase in the stock price and increasing dividend stream. * The graph measures total shareholder return, which takes into account both stock price and dividends. It assumes that dividends paid by a company are reinvested in that company’s stock. See Item 5 of the company’s latest Form 10-K filed with the U.S. Securities and Exchange Commission for those companies included in our peer group. Recently Launched Products Baqsimi is the first and only dry nasal spray to treat low Reyvow is the first new class of acute treatment for blood sugar emergencies. This is a significant improvement migraine approved in more than two decades. in user experience, exemplifies the type of innovation we Reyvow has shown the ability to provide fast and complete strive to deliver to patients and has the potential to grow pain freedom and allow adult patients to get back to the overall glucagon market. their lives. Together with Emgality, we now offer treatments for both acute and preventive care of migraine.
15 2019 INTEGRATED SUMMARY REPORT Revenue Growth Across Therapeutic Areas ($ millions, percent growth) ENDOCRINOLOGY ONCOLOGY IMMUNOLOGY NEUROSCIENCE OTHER $12,814.4 $4,614.4 $1,793.3 $1,722.9 $1,374.5 +10% +8% +57% -5% -47% Revenue in Endocrinology increased 10% primarily driven by growth of Trulicity, Basaglar and Jardiance. Oncology revenue increased 8% due to Verzenio launch in the U.S. Taltz drove the 57% revenue increase in Immunology. Neuroscience experienced a 5% decrease due to lower volume for Strattera as a result of loss of patent protection, offset in part by the launch of Emgality. Other Pharmaceutical revenue decreased 47% driven by lower volumes for Cialis, due to patent losses. Product Revenue Growth Revenue Per Employee ($ in millions represent growth in revenue excluding foreign currency impact) ($ thousands, percent growth) TRULCITY $964.5 $650 $664 $575 +13% +2% TALTZ $443.1 $490 $510 +13% +3% +4% VERZENIO $323.8 BASAGLAR $320.9 JARDIANCE $301.6 OLUMIANT $239.7 EMGALITY $157.6 2015 2016 2017 2018 2019 Seven products – Trulicity, Taltz, Verzenio, Basaglar, Jardiance, In 2019, revenue per employee increased 2% to $664,000, primarily Olumiant and Emgality – together generated revenue growth due to higher revenue driven by volume growth from Trulicity and of $2.8 billion excluding the impact of foreign currency, other new products. driven primarily by volume increases.
16 ELI LILLY AND COMPANY ANGELA MCDANIEL ASSOCIATE BRAND MANAGER, LILLY DIABETES CONNECTED CARE Modern innovations in insulin have fundamentally changed the lives of people like Angela McDaniel, who works on Lilly’s diabetes team. Angela, who has type 1 diabetes, has been able to work full-time while having three kids because modern rapid-acting insulin for mealtimes and once-a-day insulin glargine kept her blood glucose under control, even when her schedule wasn’t. “If I had been born 20 years earlier,” Angela said, “I wouldn’t have been able to have this life with children.” Angela and the millions of people with diabetes inspire us to fulfill our purpose every day. This is the power of innovative medicines, including Lilly’s modern insulins. They help people like Angela live healthier, more productive lives.
17 2019 INTEGRATED SUMMARY REPORT “If I had been born 20 years earlier, I wouldn’t have been able to have this life with children.”

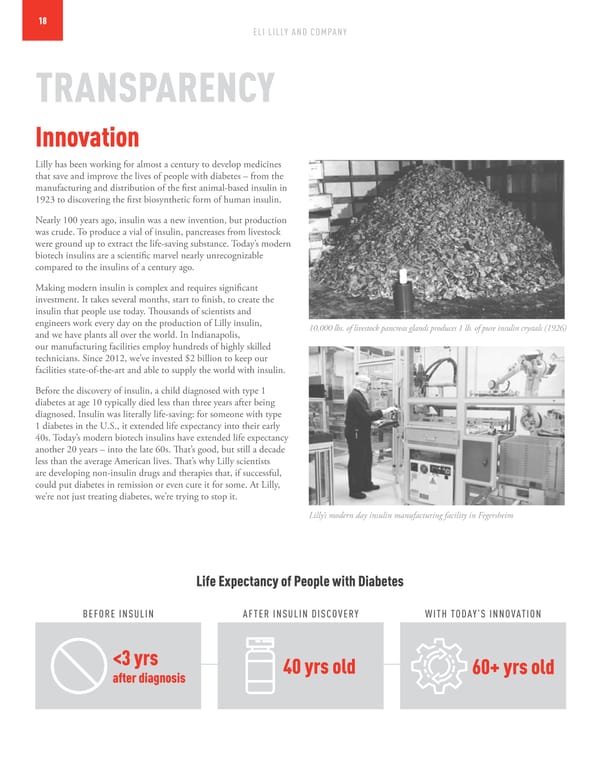
18 ELI LILLY AND COMPANY TRANSPARENCY Innovation Lilly has been working for almost a century to develop medicines that save and improve the lives of people with diabetes – from the manufacturing and distribution of the first animal-based insulin in 1923 to discovering the first biosynthetic form of human insulin. Nearly 100 years ago, insulin was a new invention, but production was crude. To produce a vial of insulin, pancreases from livestock were ground up to extract the life-saving substance. Today’s modern biotech insulins are a scientific marvel nearly unrecognizable compared to the insulins of a century ago. Making modern insulin is complex and requires significant investment. It takes several months, start to finish, to create the insulin that people use today. Thousands of scientists and engineers work every day on the production of Lilly insulin, 10,000 lbs. of livestock pancreas glands produces 1 lb. of pure insulin crystals (1926) and we have plants all over the world. In Indianapolis, our manufacturing facilities employ hundreds of highly skilled technicians. Since 2012, we’ve invested $2 billion to keep our facilities state-of-the-art and able to supply the world with insulin. Before the discovery of insulin, a child diagnosed with type 1 diabetes at age 10 typically died less than three years after being diagnosed. Insulin was literally life-saving: for someone with type 1 diabetes in the U.S., it extended life expectancy into their early 40s. Today’s modern biotech insulins have extended life expectancy another 20 years – into the late 60s. That’s good, but still a decade less than the average American lives. That’s why Lilly scientists are developing non-insulin drugs and therapies that, if successful, could put diabetes in remission or even cure it for some. At Lilly, we’re not just treating diabetes, we’re trying to stop it. Lilly’s modern day insulin manufacturing facility in Fegersheim Life Expectancy of People with Diabetes BEFORE INSULIN AFTER INSULIN DISCOVERY WITH TODAY’S INNOVATION <3 yrs 40 yrs old 60+ yrs old after diagnosis
19 2019 INTEGRATED SUMMARY REPORT CHRIS JORDAN OPERATOR, MANUFACTURING – INDIANAPOLIS DEVICE AND PACKAGING
20 ELI LILLY AND COMPANY DOUG LIEBMAN HUMALOG PATIENT BENEFITTING FROM ONE OF OUR INSULIN AFFORDABILITY PROGRAMS Doug Liebman has type 1 diabetes, so his body needs Lilly’s Humalog® insulin to convert food into energy. Doug’s health insurance plan has a high deductible, requiring him to pay $3,500 out of pocket before any insurance coverage kicks in. His bill for insulin at the start of the year is more than $1,000. But last January, Doug went to the pharmacy and his bill for four vials of Humalog was just $95. “I told the pharmacist that there was a mistake,” said Doug, 62, a gemologist in Arizona. But it wasn’t a mistake. Lilly had automatically capped Doug’s costs at $95, paying the remaining bill to Doug’s health insurance plan. The money Doug saved can help him buy new glasses more regularly, keeping his vision sharp so he can continue working.
21 2019 INTEGRATED SUMMARY REPORT “Too many people show up to the pharmacy and say, ‘I can’t afford that, just e me one vial.’ So I’m giv happy to be the one to tell them, ‘This works.’”

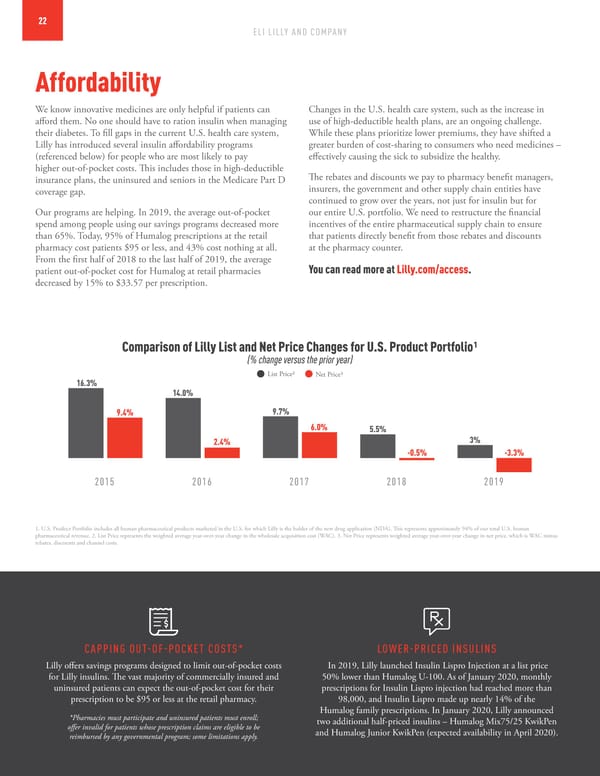
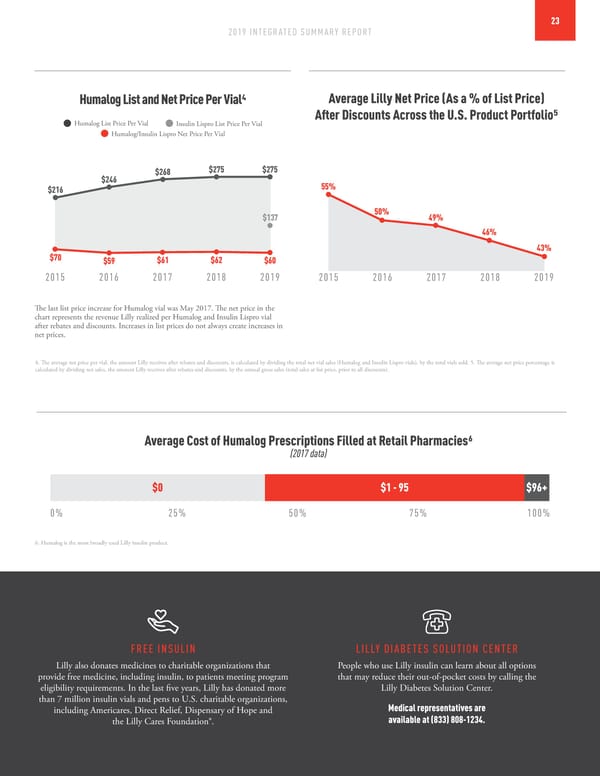
22 ELI LILLY AND COMPANY Affordability We know innovative medicines are only helpful if patients can Changes in the U.S. health care system, such as the increase in afford them. No one should have to ration insulin when managing use of high-deductible health plans, are an ongoing challenge. their diabetes. To fill gaps in the current U.S. health care system, While these plans prioritize lower premiums, they have shifted a Lilly has introduced several insulin affordability programs greater burden of cost-sharing to consumers who need medicines – (referenced below) for people who are most likely to pay effectively causing the sick to subsidize the healthy. higher out-of-pocket costs. This includes those in high-deductible The rebates and discounts we pay to pharmacy benefit managers, insurance plans, the uninsured and seniors in the Medicare Part D insurers, the government and other supply chain entities have coverage gap. continued to grow over the years, not just for insulin but for Our programs are helping. In 2019, the average out-of-pocket our entire U.S. portfolio. We need to restructure the financial spend among people using our savings programs decreased more incentives of the entire pharmaceutical supply chain to ensure than 65%. Today, 95% of Humalog prescriptions at the retail that patients directly benefit from those rebates and discounts pharmacy cost patients $95 or less, and 43% cost nothing at all. at the pharmacy counter. From the first half of 2018 to the last half of 2019, the average You can read more at Lilly.com/access. patient out-of-pocket cost for Humalog at retail pharmacies decreased by 15% to $33.57 per prescription. Comparison of Lilly List and Net Price Changes for U.S. Product Portfolio1 (% change versus the prior year) 16.3% List Price2 Net Price3 14.0% 9.4% 9.7% 6.0% 5.5% 2.4% 3% -0.5% -3.3% 2015 2016 2017 2018 2019 1. U.S. Product Portfolio includes all human pharmaceutical products marketed in the U.S. for which Lilly is the holder of the new drug application (NDA). This represents approximately 94% of our total U.S. human pharmaceutical revenue. 2. List Price represents the weighted average year-over-year change in the wholesale acquisition cost (WAC). 3. Net Price represents weighted average year-over-year change in net price, which is WAC minus rebates, discounts and channel costs. CAPPING OUT-OF-POCKET COSTS* LOWER-PRICED INSULINS Lilly offers savings programs designed to limit out-of-pocket costs In 2019, Lilly launched Insulin Lispro Injection at a list price for Lilly insulins. The vast majority of commercially insured and 50% lower than Humalog U-100. As of January 2020, monthly uninsured patients can expect the out-of-pocket cost for their prescriptions for Insulin Lispro injection had reached more than prescription to be $95 or less at the retail pharmacy. 98,000, and Insulin Lispro made up nearly 14% of the *Pharmacies must participate and uninsured patients must enroll; Humalog family prescriptions. In January 2020, Lilly announced offer invalid for patients whose prescription claims are eligible to be two additional half-priced insulins – Humalog Mix75/25 KwikPen reimbursed by any governmental program; some limitations apply. and Humalog Junior KwikPen (expected availability in April 2020).
23 2019 INTEGRATED SUMMARY REPORT Humalog List and Net Price Per Vial4 Average Lilly Net Price (As a % of List Price) After Discounts Across the U.S. Product Portfolio5 Humalog List Price Per Vial Insulin Lispro List Price Per Vial Humalog/Insulin Lispro Net Price Per Vial $268 $275 $275 $246 55% $216 $137 50% 49% 46% 43% $70 $59 $61 $62 $60 2015 2016 2017 2018 2019 2015 2016 2017 2018 2019 The last list price increase for Humalog vial was May 2017. The net price in the chart represents the revenue Lilly realized per Humalog and Insulin Lispro vial after rebates and discounts. Increases in list prices do not always create increases in net prices. 4. The average net price per vial, the amount Lilly receives after rebates and discounts, is calculated by dividing the total net vial sales (Humalog and Insulin Lispro vials), by the total vials sold. 5. The average net price percentage is calculated by dividing net sales, the amount Lilly receives after rebates and discounts, by the annual gross sales (total sales at list price, prior to all discounts). Average Cost of Humalog Prescriptions Filled at Retail Pharmacies6 (2017 data) $0 $1 - 95 $96+ 0% 25% 50% 75% 100% 6. Humalog is the most broadly used Lilly insulin product. FREE INSULIN LILLY DIABETES SOLUTION CENTER Lilly also donates medicines to charitable organizations that People who use Lilly insulin can learn about all options provide free medicine, including insulin, to patients meeting program that may reduce their out-of-pocket costs by calling the eligibility requirements. In the last five years, Lilly has donated more Lilly Diabetes Solution Center. than 7 million insulin vials and pens to U.S. charitable organizations, Medical representatives are including Americares, Direct Relief, Dispensary of Hope and available at (833) 808-1234. the Lilly Cares Foundation®.
24 ELI LILLY AND COMPANY BRANDON MARISCAL LIFE FOR A CHILD INSULIN RECIPIENT As a 5-year-old in Bolivia, Brandon Velarde Mariscal was fighting for his life. “My son was dying. He didn’t eat. He couldn’t walk,” said his mother. After more than a year, Brandon was diagnosed with type 1 diabetes. Even then, he was given too little insulin, and he continued to lose weight. Finally, Brandon’s mother found a doctor who stabilized his condition and connected them with a program to get him the insulin he needed – Life for a Child. Lilly has supplied more than 2 million vials of insulin to the program, which helps young people with diabetes in developing countries. Last year, Lilly insulin helped 19,000 young people in 31 countries. Now 24, Brandon recently graduated as a dentist and now helps improve health for others. “I want to prove that diabetes hasn’t been an impediment for me. People with diabetes, we can do it.”
25 2019 INTEGRATED SUMMARY REPORT “I want to prove that diabetes hasn’t been an or me. impediment f People with diabetes, we can do it.”

26 ELI LILLY AND COMPANY OPERATING RESPONSIBLY Corporate Responsibility We work every day to grow our business in responsible and sustainable ways that improve people’s lives, benefit society and generate positive returns for our shareholders. Pipeline Our approach to social impact starts with increasing access to our medicines and extends to improving health for people who Discovering new medicines and repurposing otherwise might not be reached by Lilly’s traditional business internal assets and legacy products for diseases model. We expand our reach by strengthening communities that disproportionately affect people living around the world and helping our employees give back. in resource-limited settings IMPROVING GLOBAL HEALTH Lilly is committed to reducing human suffering – regardless of an Programs individual’s or nation’s economic status. We leverage our resources Strengthening and creating new programs that and collaborate with leading health organizations to increase access help improve access to Lilly medicines to Lilly medicines and address complex global health challenges. Through Lilly 30x30, we’ve set a goal to improve access to quality health care for 30 million people living in settings with limited resources, each year, by 2030. We focus on three key areas: Partnerships pipeline, programs and partnerships. Building partnerships that strengthen health systems, increase access to medicines and improve care TERESA HOGAN CASE MANAGEMENT Connecting Hearts Abroad Program, Panama

27 2019 INTEGRATED SUMMARY REPORT GLOBAL HEALTH IMPACT Pipeline Asset Review Africa: Health Worker Training Initiative In 2019, we turned to our asset library in search of options to Lilly joined four other health companies and the Bill & address priority global health diseases. Our researchers examined Melinda Gates Foundation to fund community health workers more than 350 molecules and medicines alongside a list of diseases in up to six African countries. The funding will help deploy in need of better treatments. They identified about a dozen of these 2,500 community health workers equipped with digital tools. for further review and will make recommendations to a research These workers will reach an estimated 1.7 million people by 2022. governing committee in 2020. Community health workers can reduce national health Increasing Access to Lilly Medicines expenditures. Mexico: Diabetes and Pregnant Women Lilly offers more than 150 patient support programs across 50 countries that reach nearly 2 million people annually. Lilly is collaborating with the Carlos Slim Foundation to test a These programs, including new insulin affordability efforts new, less expensive screening process for pregnant women who may in the U.S., support people who are taking Lilly medicines be at risk of type 2 diabetes and gestational diabetes. The data will as well as their caregivers and loved ones. increase the understanding of both diseases within the Mexican population and inform future public policy. If fully scaled across the country, the program could reach 1.2 million women and children by 2030. Contributions at a Glance $32.6M $1.3B $90M 2019 CASH TOTAL PRODUCT TOTAL COMMITTED TO GLOBAL DONATIONS1 DONATIONS HEALTH EFFORTS THROUGH 2022 2.1M $13.4M 1.2M INSULIN VIALS RECEIVED BY 2019 UNITED WAY LILLY GLOBAL DAY OF SERVICE LIFE FOR A CHILD AS OF 2019 CONTRIBUTIONS2 EMPLOYEE VOLUNTEER HOURS3 1. Including $24.3M from the Eli Lilly and Company Foundation, Inc., a separate nonprofit organization, commonly referred to as the Lilly Foundation. 2. Including $6.9M from the Lilly Foundation. 3. Since 2008.
28 ELI LILLY AND COMPANY COMMUNITY IMPACT Beyond the impact of our medicines, Lilly has a long history of strengthening communities. We collaborate with organizations that have proven track records of social impact, and we create meaningful opportunities for our employees to give and volunteer. Disaster Relief Programs United Way When disaster strikes, Lilly and the Lilly Foundation® During our 100-year relationship with United Way, respond with donations of products and cash, including contributions from Lilly employees and retirees, plus matched contributions from Lilly employees. In advance of matching gifts from the Lilly Foundation, have totaled major storms, we partner with Direct Relief to pre-position $315 million. Together, we work to address complex insulins and other medicines. The supplies help hospitals societal challenges and create lasting change in the areas and clinics respond to immediate health needs while of health, education and financial stability. longer-term relief efforts are assessed. Connecting Hearts Abroad Global Day of Service Every year, more than 20,000 Lilly employees volunteer More than 1,200 employees have volunteered in worldwide to improve health, education and communities. communities with limited resources through our global We collaborate with local organizations in 65 countries to service program, now in its 10th year. They work on increase our impact. Together, we’ve completed thousands health-r elated projects like supporting people with diabetes of projects – from assembling cancer care packages to in South Africa and assisting refugees in Greece. Beyond the teaching in classrooms to improving local community impact they make in local communities, our employees gain centers. first-hand experience with global health challenges and Lilly’s role in addressing them. JORGE DORANTES GLOBAL EMPLOYEE COMMUNICATIONS Global Day of Service, Indianapolis

29 2019 INTEGRATED SUMMARY REPORT Environmental Highlights Our promise of making life better includes protecting and Lilly’s 2020 Environmental preserving the world we live in through our environmental and Safety Goals sustainability efforts. As our product portfolio evolves, we search for new and better ways to minimize our environmental footprint. We have already exceeded two of our 2020 environmental Progress through 2018 Goal goals – 15% reduction in phosphorous emissions and 20% improvement in waste efficiency. At the end of 2018, we had reduced phosphorous emissions in wastewater by 34.4%. This progress reflects significant efforts to phase out or minimize PHOSPHORUS EMISSIONS the use of phosphorous-based agents used to clean our 1 manufacturing process equipment. We’re now challenging ourselves IN WASTEWATER to cut an additional 10% of phosphorous emissions by the end of 2020, using 2018 as the new baseline. We’ve also improved our 34.4% REDUCTION waste efficiency by 34%, and we will now focus on achieving our recycling and waste-to-landfill targets. 15% REDUCTION Lilly recognizes the potential impacts associated with climate change and the risks of severe weather events. We set aggressive targets for improving energy efficiency and, as a result, reducing our greenhouse gas emissions intensity. 2 Through 2018, we decreased our greenhouse gas emissions WASTE EFFICIENCY intensity by 12.7% compared to our 2012 baseline. Since 2012, 34% REDUCTION eight of our 11 largest energy-consuming sites have increased production, which required additional resources. At the same time, 20% REDUCTION several of these sites improved energy performance as measured per unit of production. Overall, total energy consumption was flat compared to 2012, while our energy efficiency improved by 0.7%. Data for our 2019 performance will be shared on Lilly.com in mid-2020 in our detailed sustainability report. In 2020, 3,4 we will establish our next wave of environmental goals and start GREENHOUSE GAS EMISSIONS tracking our progress toward them. 12.7% REDUCTION In 2019, Lilly scored a rating of B on climate change and B on water from CDP. CDP is the world’s largest repository of 20% REDUCTION environmental management information. It allows companies and their stakeholders to assess environmental performance. 5 ENERGY EFFICIENCY 0.7% REDUCTION 20% REDUCTION 1. Following World Resources Institute guidance, progress toward environmental goals is reported on an adjusted basis accounting for mergers, acquisitions and divestitures, as appropriate, to ensure comparability, unless stated otherwise. 2. Per square foot of site space. 3. This goal covers Lilly’s Scope 1 and Scope 2 emissions related to site-purchased energy (e.g., electricity, steam, chilled water) and on-site fuel combustion. 4. Per unit of production or site-relevant index. Lilly’s waste goals do not include materials that are deemed “beneficially reused” without extensive processing. Examples include coal ash reused for mine reclamation or road base, and mycelia and urea reused for fertilizer. 5. In absolute terms.
30 ELI LILLY AND COMPANY Diversity and Inclusion To solve the toughest challenges in medicine, Lilly is committed management globally from 41% to 45%. For racial and ethnic to a diverse, agile workforce of top talent from around the world. minorities in the U.S., we increased management representation Everything we do comes down to our people. All of them. from 18% to 24% of total management. Diversity and inclusion (D&I) are built into the fabric of how In addition, six of the 14 members of our Executive we work – because having people with different backgrounds Committee (43%) are women, including one woman of and different voices makes our company stronger. To fulfill our color. Our 13-member board of directors includes four purpose, we must look at challenges from multiple viewpoints women (31%) and five members of underrepresented groups. and must understand the diverse experiences of the patients who With strong leadership commitment, we approached D&I as depend on us. a business-critical challenge. We conducted in-depth employee In 2019 we launched a number of D&I initiatives including research that yielded important insights about women and programs focused on people with disabilities and veterans. racial/ethnic minorities. We’re using these insights to build We’re working to improve accessibility for employees with visible more accountable leadership, more transparency in talent and invisible disabilities – earning a #2 ranking from DiversityInc management and greater cultural literacy across the workforce. in that category. We’ve also expanded efforts to hire and support Similar in-depth research is in progress to understand the veterans, who bring valuable skills to our business. Alongside these experience of LGBTQ (lesbian, gay, bisexual, transgender and efforts, our employee resource groups for people with disabilities queer/questioning) employees at Lilly. and for veterans have added new programming and extended community outreach efforts. Diversifying management ranks is also a priority, and we measure progress and strive for continued improvement. From the end of 2015 to the end of 2019, we increased the number of women in 2019 Awards and Recognitions CATALYST AWARD WORKING MOTHER NATIONAL ORGANIZATION For Our People Strategy 100 Best Companies, ON DISABILITY 25th year in a row Leading Disability Employers DIVERSITYINC BLACK ENTERPRISE ETHISPHERE Top 50 Companies for Diversity: #5 50 Best Companies for Diversity World’s Most Ethical Companies SCIENCE MAGAZINE HUMAN RIGHTS CAMPAIGN FOUNDATION FORBES Top Employers: #9 Best Place to Work for LGBTQ Equality, America’s Best Large Employers: #3, 100% score and Best Employer for Diversity
31 2019 INTEGRATED SUMMARY REPORT JOY WILLIAM INFORMATION TECHNOLOGY MARK DRESEN ALLIANCE MANAGEMENT FRANCIE SERVICE DOG AND LOYAL COMPANION
32 ELI LILLY AND COMPANY GOVERNANCE Board of Directors Q&A WITH JUAN LUCIANO, LEAD DIRECTOR What has the board done in response to shareholder engagement this year? The board believes engagement with our shareholders and other key stakeholders is central to how we govern the company. In 2019, we spoke with a number of investors on topics such as proxy access; eliminating the company’s classified board and supermajority voting requirements; environmental, social and governance topics including drug pricing and product quality and safety; our board and corporate culture; litigation and key enterprise risks; and the company’s executive compensation. As a result of input from our shareholders, we recently amended the company’s bylaws to include proxy access provisions. Once again, the board is putting forward management proposals to eliminate the classified board structure Juan, what were some of the company’s major and supermajority voting provisions. accomplishments in 2019? Why is Lilly’s current governance structure best suited In 2019, the company made major strides in implementing our to drive innovation? long-term strategy. We made significant pipeline advances. We also had significant business development engagement: we completed We’re dedicated to effective oversight of our business and believe the divestiture of Elanco Animal Health Inc., enabling Lilly to that governance is key to the board’s effective oversight of our focus solely on its human pharmaceutical business, and acquired long-term performance, strategy, key risks and compliance. Loxo Oncology, which is the largest in a series of transactions the Our board believes independence is key to strong corporate company has conducted to broaden its oncology portfolio. governance. The dual roles of an executive chair and a strong, independent lead director are important in balancing inputs to We also introduced Insulin Lispro, a lower-priced version of the board. As our Chairman and CEO, Dave Ricks provides strong Humalog®, in the U.S., providing patients with diabetes an insulin operational leadership coupled with a long-term strategic agenda. option with a list price 50% lower than the current Humalog list This is vital to our innovative research and development business, price. We’ve continued this approach in 2020, adding two versions which has prolonged product development cycles. Further, of the Humalog KwikPen® to our lower-priced insulin solutions. leveraging my experience as the CEO for a Fortune 100 company, Going forward in 2020, pricing and the dynamic health care I lead the independent directors in their analysis of company environment, particularly in the U.S., will continue to be of decisions and performance and in the other elements of our particular focus to the board. Also, the board will continue to important oversight function for the company. focus in 2020 on ensuring a robust pipeline, through both internal and external innovation, and execution on our key business priorities.
33 2019 INTEGRATED SUMMARY REPORT Congratulating Nobel Laureate Dr. William Kaelin, Jr. Lilly Board member William G. Kaelin, Jr., M.D., never had to Thanks to Dr. Kaelin and his colleagues, the world better look far to see the purpose in science. understands how cells sense and adapt to changes in oxygen. As one of the preeminent medical researchers at Harvard Medical Their transformative discovery has been integral to the School, Dr. Kaelin’s lab sits in the heart of the Dana Farber Cancer development of new therapies across a range of fields, including Institute and its waiting rooms of patients desperate for healing. anemia, heart disease and cancer. Dr. Kaelin’s late wife, Carolyn, was a beloved breast cancer surgeon, “Like most scientists, I did occasionally allow myself to dream and he has said he would often find her holding the hands of her that maybe one day this would happen,” Dr. Kaelin said. “I was patients, “sometimes while they were in tears, and she would be in overwhelmed with a sense of the moment, a sense of great tears as well.” appreciation for the life I’ve been able to lead in science, and to “You’re reminded daily that there are people counting on us,” share this wonderful recognition with the many people who have Dr. Kaelin said after winning the 2019 Nobel Prize in Physiology been a part of that life.” or Medicine. “It’s not about awards and accolades. It’s about trying We’re grateful that Dr. Kaelin is a member of our board of to get to the truth, to generate new knowledge and to have that directors. With profound curiosity and passion for solving complex knowledge eventually help our patients.” scientific challenges, he both informs and inspires our work at Lilly. CONGRATULATIONS, DR. KAELIN. THANK YOU FOR ALL YOU DO!
34 ELI LILLY AND COMPANY Board of Directors CAROLYN R. BERTOZZI, PH.D. JACKSON P. TAI J. ERIK FYRWALD Professor of Chemistry, Former Chief Executive Officer, Chief Executive Officer, Stanford University DBS Group and DBS Bank Syngenta MARSCHALL S. RUNGE, M.D., PH.D. MICHAEL L. ESKEW KATHI P. SEIFERT Executive Vice President for Medical Retired Chief Executive Officer, Retired Executive Vice President, Affairs and Medical School Dean, United Parcel Service, Inc. Kimberly-Clark Corporation University of Michigan KATHERINE BAICKER, PH.D. RALPH ALVAREZ JUAN R. LUCIANO Dean, Harris School of Public Policy, Operating Partner, Chief Executive Officer, University of Chicago Advent International Corporation Archer Daniels Midland Company KAREN WALKER JAMERE JACKSON WILLIAM G. KAELIN, JR., M.D. Senior Vice President and Chief Financial Officer, Professor of Medicine, Chief Marketing Officer, Hertz Global Holdings Inc Harvard Medical School Intel Corporation DAVID A. RICKS Chairman and Chief Executive Officer, Eli Lilly and Company
35 2019 INTEGRATED SUMMARY REPORT Board Qualifications The Board of Directors and the Corporate Governance Committee assess director candidates by considering board experience, tenure and diversity. EXPERIENCE CEO Our directors are responsible for overseeing the company’s business. Financial This fiduciary duty requires highly-skilled individuals with various qualities, attributes and professional experience. We believe the Scientific/Academic board is well-rounded, with a balance of relevant perspectives and experience, as illustrated in the chart to the right. Health care Operational/Strategic International Marketing and sales Digital BOARD TENURE 3 years or less As the chart demonstrates, our director composition reflects a mix 3-5 years of tenure on the board, which provides an effective balance of historical perspective to understand the evolution of our business 6-10 years with fresh perspectives and insights. More than 10 years DIVERSITY The board strives to achieve diversity in the broadest sense, including geography, gender, ethnicity, age and experiences. Although the board does not establish specific diversity goals or 31% 39% have a standalone diversity policy, the board’s overall diversity is an important consideration in director selection and the nomination process. The Directors and Corporate Governance Committee assesses the effectiveness of board diversity efforts. The company’s WOMEN ON UNDERREPRESENTED 13 directors range in age from 49 to 71 and include four women THE BOARD GROUPS ON THE and five members of underrepresented groups. BOARD
36 ELI LILLY AND COMPANY Board Committees of the Board of Directors All six committee charters are available online at Lilly.com/who-we-are/governance, or upon request to the company’s corporate secretary. Key responsibilities of each committee are set forth below. AUDIT COMMITTEE FINANCE COMMITTEE Reviews the company’s financial reports, systems of internal Reviews capital structure and strategies, including dividends, share control, and internal and external audit processes. It has sole repurchases, capital expenditures, investments and borrowings. authority to appoint or replace the company’s independent It makes recommendations to the board on major business auditor and assists the board’s oversight of compliance and risk development and mergers and acquisitions. It also oversees assessment and management. financial risk management policies and practices. Members: Jamere Jackson (Chair), Katherine Baicker, Members: Juan R. Luciano (Chair), Jamere Jackson, Michael L. Eskew, Jackson P. Tai, Karen Walker William G. Kaelin, Jackson P. Tai COMPENSATION COMMITTEE PUBLIC POLICY AND COMPLIANCE COMMITTEE Oversees compensation policies; establishes compensation Oversees the company’s non-financial compliance and ethics and administers benefits programs for executive officers; policies and programs. It also reviews, identifies and, and administers the deferred compensation plans, management when appropriate, brings to the attention of the board political, stock plans and incentive bonus plan. It also oversees succession social and legal trends and issues that may have an impact on management for the CEO and senior executives. the business operations, financial performance or public image Members: Ralph Alvarez (Chair), Michael L. Eskew, of the company. J. Erik Fyrwald, Kathi P. Seifert Members: Katherine Baicker (Chair), Carolyn Bertozzi, Marschall S. Runge, Karen Walker DIRECTORS AND CORPORATE GOVERNANCE COMMITTEE SCIENCE AND TECHNOLOGY COMMITTEE Identifies and recommends to the board candidates for Reviews and makes recommendations regarding the company’s membership on the board and board committees and oversees strategic research goals and objectives and pipeline of potential matters of corporate governance, director independence, new medicines. It also reviews new developments, technologies and director compensation and board performance. trends in pharmaceutical research and development and oversees Members: Michael L. Eskew (Chair), Juan R. Luciano, matters of scientific and medical integrity and risk management. Kathi P. Seifert, Jackson P. Tai Members: William G. Kaelin (Chair), Ralph Alvarez, Carolyn Bertozzi, J. Erik Fyrwald, Marschall S. Runge For more information on the Board of Directors, please see the 2020 Proxy Statement.
37 2019 INTEGRATED SUMMARY REPORT Executive Committee JOHNA L. NORTON STEPHEN F. FRY MELISSA S. BARNES Senior Vice President, Senior Vice President, Senior Vice President, Global Quality Human Resources and Diversity Enterprise Risk Management and Chief Ethics and Compliance Officer MICHAEL B. MASON DAVID A. RICKS Senior Vice President and President, Chairman and Chief Executive Officer ALFONSO ZULUETA Lilly Diabetes Senior Vice President and President, JOSHUA L. SMILEY Lilly International MYLES O’NEILL Senior Vice President and Senior Vice President and President, Chief Financial Officer ANNE E. WHITE Manufacturing Operations Senior Vice President and President, DANIEL M. SKOVRONSKY, Lilly Oncology AARTI SHAH, PH.D. M.D. PH.D. Senior Vice President and Senior Vice President and PATRIK JONSSON Chief Information and Digital Officer Chief Scientific Officer, and President, Senior Vice President and President, Lilly Research Laboratories Lilly Bio-Medicines LEIGH ANN PUSEY Senior Vice President, Not Pictured Corporate Affairs and Communications ANAT HAKIM Senior Vice President and General Counsel
38 ELI LILLY AND COMPANY CHOERI KAWILA SALES REPRESENTATIVE, DIABETES
39 2019 INTEGRATED SUMMARY REPORT Our Global Brands ENDOCRINOLOGY IMMUNOLOGY NEUROSCIENCE ONCOLOGY OTHER (tadalafil) tablets
40 ELI LILLY AND COMPANY HELPFUL LINKS Lilly News Lilly.com/newsroom Lilly’s Commitment to Corporate Responsibility and Social Impact Lilly.com/caring Lilly’s Commitment to Transparency in Our Relationships with Health Care Professionals and Health Care Organizations Lilly.com/caring/operating-responsibly/transparency Information on Lilly’s Clinical Trials Lilly.com/discovery/clinical-trials Information on the Lilly Grant Registry Lilly.com/who-we-are/lilly-grant-office Information on the Prices of Our Medicines Lillypricinginfo.com Information About Insulin Affordability LILLY DIABETES SOLUTION CENTER Insulinaffordability.com, or call toll-free (833) 808-1234 (Monday–Friday, 9 a.m.–8 p.m., Eastern time) Resources Available Via Pharmaceutical Industry Programs PHRMA’S MEDICINE ASSISTANCE TOOL MAT.org Information About Patient Assistance from a Separate Nonprofit Organization LILLY CARES FOUNDATION, INC. Lillycares.com, or call toll-free (800) 545-6962 Follow Us Eli Lilly and Company @lillypad @elilillyco Lilly.com
Eli Lilly and Company LILLY CORPORATE CENTER, INDIANAPOLIS, IN 46285 USA | 317.276.2000 | LILLY.COM





